Lu-177 labelled products
Product Offered:
Lutetium-177 DOTATATE Injection
Brand Name: SDS LU-DOTA
Product Code: LU177-DOT
Available Sizes: 3.7 GBq / 7.4 GBq (100 mCi / 200 mCi)
Shelf Life: 3 days from date of manufacture
Application: Targeted radionuclide therapy for neuroendocrine tumours (NETs) expressing somatostatin receptors.
Packing:
Each vial is supplied in a lead-shielded container, sealed and labelled with full activity, calibration date, and batch details.
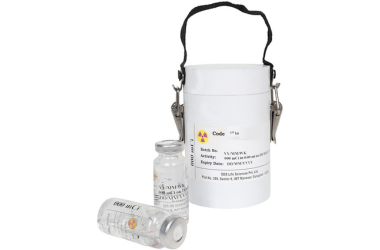
Product description:
SDS LU-DOTA is a ready-to-use radiopharmaceutical formulation of Lutetium-177 DOTATATE, designed for targeted peptide receptor radionuclide therapy (PRRT) in patients with somatostatin receptor-positive neuroendocrine tumours (NETs).
The product contains Lutetium-177 chloride (¹⁷⁷LuCl₃) complexed with DOTA-Tyr³-Octreotate (DOTATATE), forming a stable radiolabelled compound with high tumour uptake and favourable clearance kinetics.
Each batch is produced under strict GMP conditions, ensuring radiochemical purity ≥ 95%, sterility, and apparent isotonicity. The formulation is pyrogen-free and suitable for intravenous administration.
Product Operation
The injection is supplied in a Type I glass vial, shielded and ready for administration after quality verification.
Key product characteristics:
- Sterile and pyrogen-free
- Clear, colourless solution Image
- Radiochemical purity = 95%
- pH: 4.0 – 8.0
The product should be administered by qualified nuclear medicine personnel in a controlled clinical environment, following standard PRRT protocols. Unopened vials should be stored below 25°C, shielded from radiation exposure.
Product Offered:
Lutetium-177 PSMA Injection
Brand Name: SDS LU-PSMA
Product Code: LU177-PSMA
Available Sizes: 3.7 GBq / 7.4 GBq (100 mCi / 200 mCi)
Shelf Life: 3 days from date of manufacture
Packing :Each vial is enclosed in a lead-shielded container, labelled with product code, activity, calibration date, and batch number, ensuring compliance with radiation safety and transport regulations.
Application: Targeted radionuclide therapy for metastatic castration-resistant prostate cancer (mCRPC)

Product description:
SDS LU-PSMA is a therapeutic radiopharmaceutical containing Lutetium-177–labelled Prostate-Specific Membrane Antigen (PSMA) ligand, intended for targeted radionuclide therapy (TRT) in patients with metastatic prostate cancer expressing PSMA receptors.
The formulation consists of Lutetium-177 chloride (¹⁷⁷LuCl₃) complexed with a PSMA-binding ligand, forming a highly stable complex with selective tumour localisation and minimal off-target uptake.
Manufactured under GMP conditions, SDS LU-PSMA offers radiochemical purity of ≥ 95%, ensuring sterility and a pyrogen-free composition, which together guarantee safety and effectiveness for clinical use.
Product Operation
Supplied as a clear, colourless sterile solution in a Type I glass vial, the product is intended for intravenous administration by trained nuclear medicine professionals.
Product characteristics:
- Sterile and non-pyrogenic
- Radiochemical purity = 95%
- pH: 4.0 – 8.0
- Ready-to-inject, single-use vial
Storage: Keep below 25°C, shielded in a lead container, and use within 3 days of manufacture.
